Name the contractile proteins in skeletal muscle. What is the electron microscopic appearance of muscle? Write sequence of events in muscular contraction. (2011)
Myofibrils; Actin and Myosin Filaments.
Each muscle fiber contains several hundred to several thousand myofibrils. Each myofibril is composed of about 1500 adjacent myosin filaments and 3000 actin filaments, which are large polymerized protein molecules that are responsible for the actual muscle contraction. These can be seen in longitudinal view in the electron micrograph.
The thick filaments in the diagrams are myosin, and the thin filaments are actin. Note in Figure, the myosin and actin filaments partially interdigitate and thus cause the myofibrils to have alternate light and dark bands. The light bands contain only actin filaments and are called I bands because they are isotropic to polarized light. The dark bands contain myosin filaments, as well as the ends of the actin filaments where they overlap the myosin, and are called A bands because they are anisotropic to polarized light. Note also the small projections from the sides of the myosin filaments. These are cross-bridges. It is the interaction between these cross-bridges and the actin filaments that causes contraction. The actin filaments are attached to a so-called Z disc. From this disc, these filaments extend in both directions to interdigitate with the myosin filaments.
The Z disc, which itself is composed of filamentous proteins different from the actin and myosin filaments, passes crosswise across the myofibril and also crosswise from myofibril to myofibril, attaching the myofibrils to one another all the way across the muscle fiber. Therefore, the entire muscle fiber has light and dark bands, as do the individual myofibrils. These bands give skeletal and cardiac muscle their striated appearance. The portion of the myofibril (or of the whole muscle fiber) that lies between two successive Z discs is called a sarcomere. When the muscle fiber is contracted, the length of the sarcomere is about 2 micrometers. At this length, the actin filaments completely overlap the myosin filaments, and the tips of the actin filaments are just beginning to overlap one another. At this length, the muscle is capable of generating its greatest force of contraction.
What Keeps the Myosin and Actin Filaments in Place?
Titin Filamentous Molecules. The side-by-side relationship between the myosin and actin filaments is difficult to maintain. This is achieved by a large number of filamentous molecules of a protein called titin. These springy titin molecules act as a framework that holds the myosin and actin filaments in place so that the contractile machinery of the sarcomere will work.
General Mechanism of Muscle Contraction
The initiation and execution of muscle contraction occur in the following sequential steps.
1. An action potential travels along a motor nerve to its endings on muscle fibers.
2. At each ending, the nerve secretes a small amount of the neurotransmitter substance acetylcholine.
3. The acetylcholine acts on a local area of the muscle fiber membrane to open multiple “acetylcholinegated” channels through protein molecules floating in the membrane.
4. Opening of the acetylcholine-gated channels allows large quantities of sodium ions to diffuse to the interior of the muscle fiber membrane. This initiates an action potential at the membrane.
5. The action potential travels along the muscle fiber membrane in the same way that action potentials travel along nerve fiber membranes.
6. The action potential depolarizes the muscle membrane, and much of the action potential electricity flows through the center of the muscle fiber. Here it causes the sarcoplasmic reticulum to release large quantities of calcium ions that have been stored within this reticulum.
7. The calcium ions initiate attractive forces between the actin and myosin filaments, causing them to slide alongside each other, which is the contractile process.
8. After a fraction of a second, the calcium ions are pumped back into the sarcoplasmic reticulum by a Ca++ membrane pump, and they remain stored in the reticulum until a new muscle action potential comes along; this removal of calcium ions from the myofibrils causes the muscle contraction to cease.
The molecular machinery of the muscle contractile process.
Sliding Filament Mechanism of Muscle Contraction
Sliding is caused by forces generated by interaction of the cross-bridges from the myosin filaments with the actin filaments. Under resting conditions, these forces are inactive, but when an action potential travels along the muscle fiber, this causes the sarcoplasmic reticulum to release large quantities of calcium ions that rapidly surround the myofibrils. The calcium ions in turn activate the forces between the myosin and actin filaments, and contraction begins. But energy is needed for the contractile process to proceed. This energy comes from high-energy bonds in the ATP molecule, which is degraded to adenosine diphosphate (ADP) to liberate the energy. In the next few sections, we describe what is known about the details of these molecular processes of contraction.
ATP as the Source of Energy for Contraction—
The following sequence of events is believed to be the means by which this occurs:
1. Before contraction begins the heads of the cross bridges bind with ATP. The ATPase activity of the myosin head immediately cleaves the ATP but leaves the cleavage products, ADP plus phosphate ion, bound to the head. In this state, the conformation of the head is such that it extends perpendicularly toward the actin filament but is not yet attached to the actin.
2. When the troponin-tropomyosin complex binds with calcium ions, active sites on the actin filament are uncovered, and the myosin heads then bind with these.
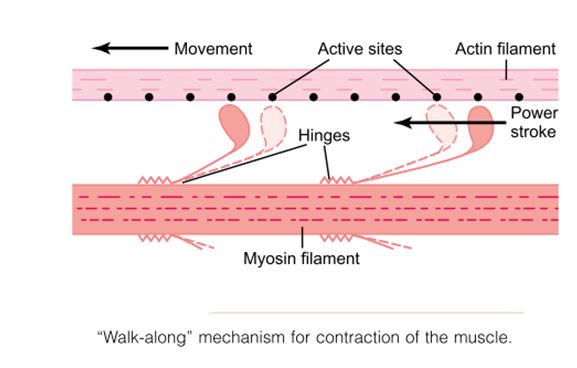
Myosin filament “Walk-along” mechanism for contraction of the muscle.
One hypothesis for which considerable evidence exists is the “walk-along” theory (or “ratchet” theory) of contraction. The heads of two cross-bridges attach to and disengage from active sites of an actin filament. It is postulated that when a head attaches to an active site, this attachment simultaneously causes profound changes in the intramolecular forces between the head and arm of its cross-bridge. The new alignment of forces causes the head to tilt toward the arm and to drag the actin filament along with it. This tilt of the head is called the power stroke. Then, immediately after tilting, the head automatically breaks away from the active site. Next, the head returns to its extended direction. In this position, it combines with a new active site farther down along the actin filament; then the head tilts again to cause a new power stroke, and the actin filament moves another step. Thus, the heads of the cross bridges bend back and forth and step by step walk along the actin filament, pulling the ends of two successive actin filaments toward the center of the myosin filament.
Each one of the cross-bridges is believed to operate independently of all others, each attaching and pulling in a continuous repeated cycle. Therefore, the greater the number of cross-bridges in contact with the actin filament, at any given time, the greater, the force of contraction.
3. The bond between the head of the cross-bridge and the active site of the actin filament causes a conformational change in the head, prompting the head to tilt toward the arm of the cross-bridge. This provides the power stroke for pulling the actin filament. The energy that activates the power stroke is the energy already stored, like a “cocked” spring, by the conformational change that occurred in the head when the ATP molecule was cleaved earlier.
4. Once the head of the cross-bridge tilts, this allows release of the ADP and phosphate ion that were previously attached to the head. At the site of release of the ADP, a new molecule of ATP binds. This binding of new ATP causes detachment of the head from the actin.
5. After the head has detached from the actin, the new molecule of ATP is cleaved to begin the next cycle, leading to a new power stroke. That is, the energy again “cocks” the head back to its perpendicular condition, ready to begin the new power stroke cycle.
6. When the cocked head (with its stored energy derived from the cleaved ATP) binds with a new active site on the actin filament, it becomes uncocked and once again provides a new power stroke.
Thus, the process proceeds again and again until the actin filaments pull the Z membrane up against the ends of the myosin filaments or until the load on the muscle becomes too great for further pulling to occur.